College of Medicine Researcher Focuses on Immunotherapy for Cancer, Autoimmune Disease, and COVID-19
March 2022
By Kathleen Snoeblen
From a very young age, UCF Assistant Professor Hung Nguyen saw how his father, a physician, and his mother, a nurse, worked tirelessly to take care of patients. “My parents worked so hard helping people and that’s why my wife and I were inspired to work in the medical field,” said Nguyen.
Yet, with a drive to help as many people as he can and an inquisitive, adventurous nature, Nguyen took a slightly different path than his parents. He chose biochemical and pharmaceutical research rather than medicine because of its potential for discovering new treatments and drug therapies. “With research, we can save a thousand lives if we can do it well,” Nguyen said.
For the past 10 years, he has rooted his career in the study of cellular metabolism and immunology, focusing on how changes in a person’s metabolism affect their immune cells and how those cells, in turn, respond to diseases.
“I think the cell is like a human. We are made by cells. The cells enjoy their own food” Nguyen said. “They have their own exercise. They also have breath, their own air. They communicate with each other. And why we care about metabolism is because what we eat, the cell will eat. The food will decide our health. That’s very, very clear.
“The cell metabolism decides the body functions and the fueling in us,” Nguyen said. “For example, the immune cells are metabolized differently from other cells like the nervous system cells. So, if the nervous system cells metabolize well—they have normal metabolism—you will function well, you’re happy, the cells are happy.”
Nguyen joined UCF’s College of Medicine (COM) more than two years ago. In his lab at the Burnett School of Biomedical Sciences, he leads three research projects. Nguyen seeks to find ways to improve immunotherapy for cancer, treat Graft-versus-Host Disease, and combat COVID-19 (coronavirus disease 2019). Through his research, Nguyen has developed a technology to help identify biomarkers about a patient’s cellular metabolism and their immune response to COVID-19. “Doctors can use the information to help them decide the treatment therapy for the patient and to predict how the patient can recover,” he said. The technology offers a novel approach for predicting, treating and triaging infected patients.
A Single-Cell Immunometabolic Assay to Predict the Severity of COVID-19
While investigating the relationship between cellular metabolism and COVID-19-induced immunological dysfunction, Nguyen found that he needed a more effective, sensitive assay than those readily available. The team also wanted to determine metabolic biomarkers for predicting the prognosis of COVID-19 patients and enable doctors to determine the treatment therapy for those patients.
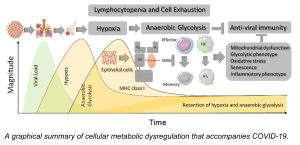
They focused on the metabolism of immune cells in COVID-19 patients that develop lymphocytopenia, a disorder in which a person’s blood doesn’t have enough white blood cells, called lymphocytes, to fight off infection. Nguyen noted that individuals with pre-existing metabolic comorbidities, including obesity, hypertension, diabetes, and cardiovascular disease, are at a far higher risk of suffering severe complications from COVID-19.
“We are the first group to identify that glucose metabolism is one of the risk factors that links to severe COVID-19 severity infection,” he said. The team recognized that patients who yield high blood glucose during virus infection were not recovering normally because the immune cells in their bodies become dysfunctional.
According to Nguyen, cellular metabolism plays a critical role in dictating T cell responses during severe COVID-19. “In our bodies, we have innate immunity and adaptive immunity. Natural Killer (NK) cells belong to innate immunity, and lymphocytes, such as CD8- and CD4- T cells, belong to adaptive immunity. “Normally, in the immune response to a viral infection, the first responders are NK cells,” he said. “The second response belongs to the T cell immunity. In the second phase, after the cells respond, those cells become memory cells. They remember the infectious factor or invaders so they can respond better the next time,” he noted.
“But why is it that with COVID-19, you don’t have enough memory cells to respond again?” Nguyen questioned. With re-exposure to a virus, a patient should see an increase in memory cells, allowing that patient to recover again. However, Nguyen and his team found that patients with severe COVID didn’t have or make enough memory cells. “That’s why their response was weak,” he said. “CD8 memory T cells and memory NKs are two very important cells that remember infection-fighting.
Thus, Nguyen developed the assay, which provides a comprehensive single-cell metabolic landscape of the antiviral response of immune cells in patients fighting SARS-CoV-2 (the virus that causes the COVID-19 disease). The assay captures the immune response in both the blood and lungs of a patient. “We needed to read out the functionality of a patient’s cell metabolism using a small number of the cells,” he said. The team also found that traditional technologies couldn’t provide readouts of the functionality for many different cells at the same time. “We wanted to understand not only one cell subset; we wanted to understand various cell subsets and in the context of COVID-19.”
He and the team worked long hours evaluating the immune response in blood samples from patients with severe COVID-19. For 4-1/2 months, late into the evenings, with the support from Dr. Gardell and Daniel Lupu at AdventHealth, they collected and processed samples from ICU patients “We had to
process each sample immediately, the same day because if we kept a sample outside for long, the metabolism would keep changing.”
With the new tool, they found that memory cells don’t eat sugar, glucose. “They eat different food [fatty acids]. So, if your blood glucose level is so high, they don’t eat that, and they die. That’s why you can see in obesity, in heart disease, metabolic disorder patients who have high glucose levels in the blood. Or they have a different kind of nutrition for the memory cells to use. That’s why they have trouble with responding to the virus.”
Besides providing an immune cell landscape, the assay is an effective tool to identify biomarkers for predicting the severity of COVID-19 and enables his team to identify novel immune cell subsets that exhibit metabolic dysfunction, too. Those subsets also serve as predictive biomarkers for COVID-19 severity or targets for therapeutic interventions in COVID-19 disease. For more information about the tool and the team’s findings, see the technology sheet.
What’s Next?
In the next phase of his research, Nguyen plans to use the assay to screen for drugs that can potentially reprogram the metabolism so that high-risk patients can maintain adequate amounts of memory cells to combat infection. He also wants to improve the assay “because we want to understand how the cell breathes, and how the cell eats and secretes.” For that phase, he is collaborating with UCF COM researcher Justine Tigno-Aranjuez, Ph.D., targeting glycolysis to control COVID-19.
“We also want to use our knowledge to further induce the vaccine response and generate a drug that directly treats the acute SAR-COV-2 infection,” he said.
This technology is available for licensing. Contact Benjamin Neymotin, Ph.D., for more information.