Paralyzing Breast Cancer, Stopping Metastasis in its Tracks
Tech Blog Thursday mixes serious science with humor and easily recognizable analogies for the less-than-scientifically-inclined. The purpose of this blog series is to illustrate the potential of not-yet-commercialized technology and encourage excitement about the possibilities.
October 17, 2014
Dear Cancer, I hope one day you are just a zodiac sign. 
–Anonymous
For many breast cancer patients and survivors, cancer recurrence and metastasis, the spreading of the cancer to other sites, are constant concerns. In fact, an estimated 90% of breast cancer- related deaths are due to metastasis. Once they break away from the original tumor, metastatic breast cancer cells can spread to other parts of the body, and in particular, the bone, brain, liver, and lung. Cell movement represents a significant challenge for cancer researchers and oncologists; and currently, there is no cure for metastatic (stage IV) breast cancer. For these patients, treatment options are typically limited to systemic “whole body” therapies such as chemotherapy, which can have toxic side effects on healthy cells.
Cancer metastasis is a complex process which requires significant rearrangement of a cellular support structure known as the cytoskeleton (an intricate network of filaments akin to the skeletal system, but less rigid and more dynamic). Because of its critical role in cell movement, the cytoskeleton has become a target for many cancer drugs in development. Widespread use of these drugs, however, is limited by their high toxicity. At the UCF College of Medicine’s Burnett School of Biomedical Sciences, Dr. Annette Khaled and her research team are developing a new anti-cancer compound called CT20p. CT20p specifically disrupts the cytoskeleton of breast cancer cells, without harming healthy cells. In response to treatment with CT20p, breast cancer cells detach from their surroundings, round up, stop moving, and die – similarly to what would happen if we suddenly lost the structure and support provided by our bones.
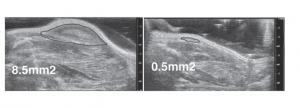
Breast cancer tumors were implanted in mice. CT20p treatment caused tumor regression (right figure).
To safely deliver CT20p to the target cancer cells, CT20p is encapsulated in a specialized nanoparticle developed by Dr. J. Manuel Perez at the UCF NanoScience Technology Center. The surfaces of the nanoparticles contain ligands that specifically bind to cancer cells, concentrating the CT20p at those target sites. As proof of concept, injection of the CT20p-nanoparticle complex into a breast cancer mouse model caused significant tumor regression and did not damage the liver or the spleen, two sites where untargeted nanoparticles could accumulate. The complex is currently being tested with human breast cancer samples.
“Our goal is to develop CT20p into a powerful cancer therapeutic to kill the most invasive types of breast cancer cells that are responsible for cancer recurrence and metastasis. Successful completion of our work will have a significant impact on the survivability of metastatic breast cancer patients,” says Dr. Khaled.
As a new therapeutic, CT20p could be used to treat early-stage breast cancer as well as advanced metastatic breast cancer, the fatal form of the disease. For all breast cancer patients, our hope is that, one day, we will be able to stop cancer dead in its tracks.
Learn more about partnering with UCF to bring this breast cancer therapeutic to market, contact Brion Berman.
>>Guest blog post by Christina Kittipatarin, Ph.D.